Stem Cell Technology
Stem cells retain the capacity to self-renew as well as to produce progeny with a restricted mitotic potential and restricted range of distinct types of differentiated cells they give rise to. The formation of blood cells, also called haematopoiesis, is the classical example of the concept of stem cells. Indirect assay methods were developed to identify the haematopoietic stem cells. The process of haematopoiesis occurs in the spleen and bone marrow in mice. In human beings, about 100,000 haematopoietic stem cells produce one billion RBCs, one billion platelets, one million T-cells, and one million B-cells per kg body weight per day.
Stem Cell Research
Stem cells are the raw material from which all of the body’s mature, differentiated cells are made. Stem cells give rise to brain cells, nerve cells, heart cells, pancreatic cells, etc. They have the potential to replace cell tissue that has been damaged or destroyed by severe illnesses. They can replicate themselves over and over for a very long time. Understanding how stem cells develop into healthy and diseased cells will assist the search for cures.
There are two types of Stem Cells
Embryonic (also called “pluripotent”) stem cells
Embryonic stem cells are capable of developing into all the cell types of the body. There are two sources of embryonic stem cells:
1) Excess fertilized eggs from IVF (in-vitro fertilization) clinics can be used as a source of embryonic stem cells. Tens of thousands of frozen embryos are routinely destroyed when couples finish their treatment. These surplus embryos can be used to produce stem cells. Regenerative medical research is finding modern methodology to develop these cells into new, healthy tissue to treat severe and often fatal illnesses.
2) Therapeutic Cloning (Somatic Cell Nuclear Transfer)
In Somatic Cell Nuclear Transfer, the nucleus of a donated egg is removed and replaced with the nucleus of a mature, "somatic cell" (a skin cell, for example). No sperm is involved in this process, and the embryos are not created to be implanted in a woman’s uterus. The resulting stem cells can be induced to develop into specialized cells that are useful to treat dangerous illnesses.
Adult stem cells
Adult stem cells are less versatile and more difficult to identify, isolate, and purify.
The stem cells are extracted from a 5-7 day old blastocyst. Stem cells can divide in culture to form more of their own kind, thereby creating a stem cell line. Later, these are induced to generate healthy tissue needed by patients.
The Importance of Stem Cell Therapy
Stem cells allow us to study how organisms grow and develop over time. Stem cells can replace diseased or damaged cells that cannot heal or renew themselves. To develop and research and find new drugs and medicines, stem cells can be used to test these chemicals and drugs. Stem cells help us to understand what is called the “genetic machinery.” Tremendous efforts are already ongoing to treat diseases like Parkinson’s Disease, Leukemia (Bone Marrow Transplants), diabetes, multiple sclerosis, etc., using stem cell therapy. Another area of importance is to regenerate tissues to be used as skin grafts to treat patients suffering from severe burns.
The Controversy regarding Stem Cell Therapy
The supporters of Stem Cell Therapy argue that embryonic stem cell research (ESCR) fulfills the ethical obligation to alleviate human suffering. The end justifies the means. If the research is directed towards making the human species disease and pain-free, any kind of research including stem cell research should be allowed and pursued. They further argue that as excess IVF embryos will be discarded anyway, they should better be used in research. As far as Stem Cell Nuclear Transfer (SCNT) or Therapeutic Cloning is concerned, it produces cells in a petri dish, not out of pregnancy.
Those who oppose the stem cell therapy accuse the researchers involved in this as murderers. Their argument is that extracting the stem cells from a human blastocyst leads to the destruction or killing of the embryo, which amounts to murdering, or killing a potential human life. Further, there is a risk of commercial exploitation of the couples who willingly or unknowingly become participants in ESCR. Some opponents also predict that in the future this will lead to reproductive cloning.
It is not easy to decide or resolve the serious ethical issues surrounding this area of research. Often the question is asked as to at what stage of embryonic development the blastocyst should be regarded as a “person with life.” Often the blastocyst used in stem cell research is microscopically so small with no nervous system, that the supporters of stem cell therapy do not consider it as living. There is also a conflict between embryonic stem cell research science and religion, as most of the major world religions do not support using embryos for research. There have been major hurdles in creating and formulating a human public policy on this issue due to the sensitive nature of the problem.
Several methods have been developed to study haematopoiesis and stem cells:
a) Repopulation assay - Edmens Snell’s group created mice which were genetically identical by mating of sibling mice after 21 generations. Two groups of mice were lethally X-irradiated to destroy their blood cell-forming capacity. One of these groups was injected with marrow cells from the femur bone of a normal and healthy albino mouse. It was observed that this group survived whereas the mice in the other group died. The spleen of mice which survived had the colonies of the bone marrow cells just like bacterial colonies on a Petri plate. This came to be known as colony forming units of spleen (CFU-S) and the technique is known as repopulation assay.
b) The in vitro clonal assay - In this assay, the stem cells proliferate to form colonies of differentiated cells on semi-solid media. This assay helps in identifying growth factors required for the formation of blood cells from the primitive stem cells. One of the first commercialized biotechnology products – erythropoietin – was assayed by this procedure.
c) Long term marrow culture - In this method, the marrow cells from the femur bone were grown under in vitro conditions on plastic surfaces. These techniques were helpful in bone marrow transplantation and treatment of blood cancer by releasing immature blood cells into the bloodstream.
d) Embryonic stem cell culture - Embryonic stem cells are cell lines derived from the inner cell mass of a fertilized mouse embryo without the use of immortalizing or transforming agents. The Inner Cell Mass (ICM) are the cells that are maintained in tissue culture in the presence of irradiated fibroblast cells. These cells are often used in creating chimeric mice. In 1998, J.A. Thomson developed the method to multiply human embryonic stem cells. Human ICM can now also be derived either by IVF or from germ cell precursors and cultured on a Petri plate. The differentiation of these cells into lineage-restricted (neuronal and glial) cells can be accomplished by altering the media in which the cells grow.
e) The ICM cells could be used to create chimeric mice - In chimeric mice it was possible to take ES cells from a black mouse and implant them into the embryo of an albino mouse (white). The progeny so developed had skin color of black and white (a chimera).
Following diagram shows the scheme of obtaining chimeras.
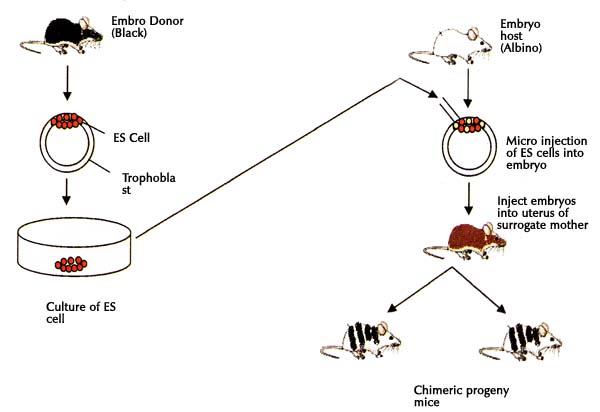
Genetic Engineering of Animal Cells and Their Applications
The mammalian cells are genetically modified by introducing the genes needed for specific purposes such as production of specific proteins or to improve the characteristics of a cell line. The methods used to introduce the foreign genes/DNA into mammalian cells are: Electroporation, Lipofection, Microinjection and/or fusion of mammalian cells with bacteria or viruses.
After the integration of the foreign DNA into the mammalian cells, the transfected/transformed cells are selected by using suitable markers. Some of such markers in use are: Viral thymidine kinase, Bacterial dihydrofolate reductase, Bacterial neomycin phosphotransferase. It has been possible to overproduce several proteins in mammalian cells through genetic manipulations e.g. tissue plasminogen activator, erythropoietin, interleukin-2, interferon-beta, clotting factors VIII and IX, tumor necrosis factors. The recombinant mammalian cells are also conveniently used for the production of monoclonal antibodies.
Manipulation of Gene Expression in Eukaryotes
The eukaryotic organisms have the capability to bring about the post-translational modifications such as glycosylation, phosphorylation, proteolytic cleavage etc., which ultimately helps in the production of stable and biologically active proteins. Due to these reasons, the use of eukaryotic expression system is preferred; however, it is difficult to conduct experiments with eukaryotic cells. The introduction of a foreign DNA into animal cells is called transfection. The insert DNA in the eukaryotic cells may be associated with vector or integrated into the host chromosomal DNA.
Among the various hosts used for the expression of cloned genes, the common yeast Saccharomyces cerevisiae is the most extensively used. Besides this, the cultured insect cells are in use for expressing cloned DNAs. Baculoviruses exclusively infect insect cells. The DNA of these viruses encodes for several products and their productivity in cells is very high — up to more than 10,000 times compared to mammalian cells. The baculoviruses not only carry a large number of foreign genes but can also express and process the products formed.
By using baculovirus as an expression vector system, a good number of mammalian and viral proteins have been synthesized. The most commonly used baculovirus is Autographa californica multiple nuclear polyhedrosis virus (AcMNPV). It grows on the insect cell lines and produces high levels of polyhedrin or a recombinant protein.
The mammalian cell expression vectors are used for the production of specific recombinant proteins and to study the function and regulation of mammalian genes. However, large-scale production of recombinant proteins with engineered mammalian cells is costly. The mammalian vector contains a eukaryotic origin of replication from an animal virus such as Simian virus 40 (SV40) and a prokaryotic origin of replication. It has a multiple cloning site and a selectable marker gene, both of which remain under the control of eukaryotic promoter and polyadenylation sequences.
These sequences are obtained from either animal viruses (SV40, herpes simplex virus) or mammalian genes (growth hormone, metallothionein). The promoter sequences facilitate the transcription of cloned genes (at the multiple cloning site) and the selectable marker genes. On the other hand, the polyadenylation sequences terminate the transcription.
Collection and Purification Process of Recombinant Proteins
As the recombinant proteins start accumulating in the host cells, it becomes important to collect and purify them. This is a tricky process since many times the recombinant protein is a foreign body for the host cells and the enzyme machinery of the host cell becomes activated to degrade the outside protein. One of the strategies adopted is the use of bacterial strains deficient in proteases or, alternatively, the recombinant proteins are fused with the native host proteins. The fusion proteins are resistant to protease activity. Sometimes, the foreign proteins accumulate as aggregates in the host organism which minimizes the protease degradation. The best way out is to quickly export and secrete out the recombinant proteins into the surrounding medium. The recovery and the purification of foreign proteins is easier from the exported proteins. Efforts have been made to develop methods to increase the export of recombinant proteins.
Some of the species of the bacterium Bacillus subtilis normally secrete large quantities of extracellular proteins. A short DNA sequence called signal sequence from such species is introduced into other B. subtilis. These bacteria produce recombinant DNA tagged with signal peptide, which promotes export and secretion. This signal peptide is removed after the purification of the foreign protein. The techniques used for the purification of recombinant proteins from the mixture of secreted proteins are affinity tagging, immunoaffinity purification, etc.
Organ Culture and Histotypic Cultures
The cell-cell interaction leads to multistep events in in vivo situations. For example, hormone stimulation of fibroblasts is responsible for the release of surfactant by the lung alveolar cells. Androgen binding to stromal cells stimulates the prostate epithelium. In other words, hormones, nutritional factors, and xenobiotics exert stimulating effects on the cells to function in a coordinated manner. Xenobiotics broadly refer to unnatural, foreign, and synthetic chemicals such as pesticides, herbicides, refrigerants, solvents, and other organic compounds.
It is impossible to study these cellular interactions that occur in the in vivo system with isolated cells or cells in culture. This has led to the attempts to develop organ and histotypic cultures with the aim of creating in vitro models comparable to the in vivo system. The three types of such cultures are:
a) Organ culture – In this type of culture, the whole organs or small fragments of the organs with their special and intrinsic properties intact are used in culture.
b) Histotypic culture – The cell lines grown in three-dimensional matrix to high density represent histotypic cultures.
c) Organotypic cultures – A component of an organ is created by using cells from different lineages in proper ratio and spatial relationship under laboratory conditions.
Organ Culture
In the organ culture, the cells are integrated as a single unit which helps to retain the cell-to-cell interactions found in the native tissues or organs. Due to the preservation of structural integrity of the original tissue, the associated cells continue to exchange signals through cell adhesion or communications. However, due to the lack of a vascular system in the organ culture, the nutrient supply and gas exchange of the cells become limited. To overcome this, organ cultures are placed at the interface between the liquid and gaseous phases. Sometimes, exposure to high O2 concentrations may lead to oxygen-induced toxicity. Due to the inadequate supply of nutrients and oxygen, necrosis at the central part of the organ may occur. In general, organ cultures do not grow except for some amount of proliferation in the outer cell layers.
Techniques and Procedure for Organ Culture
To optimize nutrient and gas exchanges, tissues are kept at a gas-liquid interface using support materials such as semisolid gel of agar, clotted plasma, micropore filter, lens paper, or strips of Perspex or plexiglass. Organ cultures can also be grown on stainless steel grids. Filter-well inserts made of ceramic, collagen, or nitrocellulose are popular choices and have been successfully used to develop functionally integrated thyroid epithelium, stratified epidermis, intestinal epithelium, and renal epithelium.
Procedure:
a) The organ tissue is collected after dissection.
b) The size of the tissue is reduced to less than 1 mm in thickness.
c) The tissue is placed on a gas-medium interface support.
d) Incubation in a CO2 incubator.
e) M199 or CMRL 1066 medium is used and changed frequently.
f) Techniques such as histology, autoradiography, and immunochemistry are used to study the cultures.
Advantages of Organ Culture
Organ cultures allow the study of integrated tissue behavior in the laboratory and help in understanding the biochemical and molecular functions of an organ/tissue.
Limitations of Organ Culture
It is a difficult and expensive technique with high variation and low reproducibility. Each experiment requires a new or fresh organ, as organ cultures are not propagated.
Histotypic Cultures
Histotypic culture allows dispersed monolayers to regenerate tissue-like structures through growth in a three-dimensional matrix at high cell density. Techniques used include:
a) Gel and Sponge Technique – Collagen gels or gelatin sponges provide a matrix for morphogenesis and cell growth. Cells penetrate and grow within these structures.
b) Hollow Fibers Technique – Hollow fibers enhance nutrient and gas exchange. Perfusion chambers with beds of plastic capillary fibers have been developed where cells attach and grow to form tissue-like structures.
c) Spheroids – Reassociation of dissociated cultured cells forms clusters called spheroids, mimicking embryonic reassembly into specialized structures. Cells self-sort into tissue-like architectures. However, nutrient and gas diffusion is limited.
d) Multicellular Tumor Spheroids (MCTS) – Used as in vitro models for tumor studies, including drug testing, immune response, and gene therapy. A size >500 µm can result in necrosis at the center. MCTS are formed by treating monolayer/aggregated tumor cells with trypsin, inoculating them into magnetic stirrer flasks or roller tubes. Within 3-5 days, spheroids form. They are measured regularly and used to model tumor growth and study drug effects and disease spread.
Organotypic Cultures
These cultures are used to develop certain tissues or tissue models for example skin equivalents have been created by culturing dermis, epidermis and intervening layer of collagen simultaneously. Similarly models have been developed for prostrate, breast etc. Organotypic culture involves the combination of cells in a specific ratio to create a component of an organ.