Patents
A patent gives its owner a monopoly right to make and sell or use the patented product or process of an invention for a limited period of time. A Patentable matter should meet certain criteria such as
a) utility,
b) novelty,
c) statutory subject matter.
The laws pertaining to patency may vary from country to country. A patented pharmaceutical product in USA, has to undergo a regulatory purview by Food and Drug Administration (FDA) before its clinical use is allowed. According to the U.S. Federal Insecticide, Fungicide, and Rodenticide Act, before the release of genetically engineered microbial pesticides, it is customary to obtain a permit from the Environmental Protection Agency (EPA). The Indian Patents Act of 1970 allows “process patents but no "product patents" for foods, chemicals, drugs and pharmaceuticals. Patents are granted or complaints of infringement of these patents decided by courts in accordance with the patent law of the concerned country.
A patent consists of three parts: the grant, specifications, and claims.
a) The Grant- The grant is filled at the patent office which is not published. It is the actual signed document which is the agreement that grants patent rights to the inventor.
b) Specifications- The specifications part is narrative and describes the subject matter and explains about the process of invention. It is published as a single document which is made public at a minimum charge from the patent office.
c) The claim - The claim section specifically defines the scope of the invention to be protected by the patent to which the others may not practice.
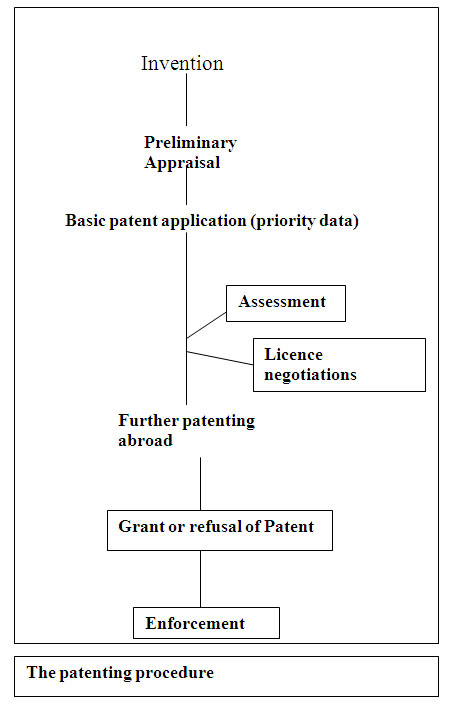
Reading a Patent
In order to file a patent, the documents required should have a specialized structure. An applicant should first file a patent in his or her own country and then at a later stage/date files in the international office. The application is prepared with a specific, clear and concise title. A patent attorney is appointed for the legal aspects of the patent whose job also involves filling the patent in the office of the Controller of Patents.
Patenting process varies from country to country. Therefore, it is effective only within the jurisdiction of that country which grants the patent. It is interesting to note that a patent application rejected in one country may be given patent in another country. A biotechnology company, Genetech, applied for a patent in the U.K. for the production of human tissue plasminogen activator (tPA) by recombinanat DNA process. The application was rejected by the U.K. Patent office but they were awarded the patent rights for t-PA production in USA and Japan. After appeals and legal battles, patent was finally granted in UK also.
According to the International conventions, one cannot patent certain things such as a) the use of drugs, antibiotics or vaccines for any form of diagnosis, prevention or cure of diseases; b) artificial insemination, and c) in vitro fertilization and embryo transfer.
The advantages of patents and other forms of IPR are:
(c) These practices help in encouraging and safeguarding intellectual and artistic creations.
(d) They help to disseminate new ideas and technologies quickly and widely.
(e) They promote investment.
(f) They help the inventor to provide the fruits and benefits of his creation and invention to the public.
(g) They provide increased opportunities for the distribution of the above effects across countries in a manner proportionate to national levels of economic and industrial development (OECD, Paris, 1989)
The EPO has suggested to patent the genetically engineered liveforms. “Onco mouse” is one of the examples of which initially the patent claims was rejected but on appeal the previous decision was overruled. Genetically engineered E.coli, in which human genes for insulin, growth hormone, t-PA etc have been introduced, have been patented in the USA. The transgenic herbicide and bollworm resistant cotton, and insect- resistant tobacco have been granted patents.
The introduction of the concept of “patenting” to biotechnology has added a financial angle to the area of research. In the past, research used to be mostly for academic interest and worthwhile scientific contributions. The main support and funding used to be provided by the Government/Universities/Institutes. With the financial support of the private companies, the scenario has changed considerably. When the companies finance any project, they do expect some returns for their investment which adds the concept of financial gains also besides the academic recognition for the researcher. The consequences are, that some people carry out research in secrecy and opt for patenting of their discoveries rather than publishing.