Treatment of diseases
Manufacturing of Pharmaceutical Drugs Through Biotechnology
Various drugs are being manufactured using biotechnology to treat different diseases. Using gene therapy, two drugs –Insulin and Interferons have already been produced. Insulin is used to treat diabetes and Interferon is used for the treatment against some tumour viruses. It is possible to manufacture these drugs in large quantities by cloning the corresponding genes from human or animals through plasmid vectors in bacteria. This procedure brings down the cost of manufacturing the drugs. Lots of research is also going on a number of proteins such as urokinase, factor VII:C, human growth hormone (HGH) etc. The Human Growth hormone for treating dwarfism due to hypopitiuitary activity was synthesized using recombinant DNA technique and was commercially introduced under the market name of prototropin in USA and somatonorm in Britain. Both Insulin and growth hormone were manufactured under license from Genetech Inc. based in USA.
The transgenic animals are being used to produce products that are being now commercially use in the area of human health and medicine. In 2009, the US Food and Drug Administration approved the drug ATryn, which is the first human biological drug produced in goat. It is now being commercially manufactured and marketed by GTC Biotherapeutics, a Massachusetts based biotech company. ATryn is an anticoagulant with the property of dissolving the blood clots during surgery or child birth.
It is extracted from the goat’s milk. The method of microinjection was used to insert the human antithrombin genes in to the cell nucleus of the goat’s embryo. The drug is made from the milk of these transgenic goats as the gene is expressed in the milk of the animals. The transgenic goats can produce the same amount of antithrombin in a year as 90,000 blood donations.
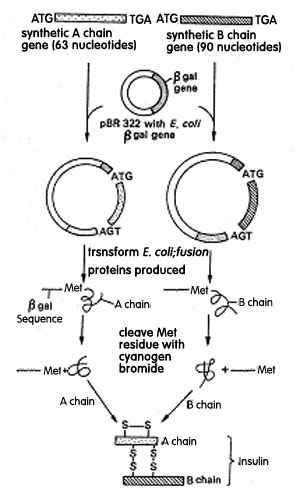
Steps used for the synthesis of insulin from cloned DNA segment
Gene therapy
If a child or an embryo is diagnosed to carry a defective gene leading to disability, following methods are used to correct this defect:
a) replacement of defective gene with a normal gene.
b) correction of the defective gene through gene targeting.
c) gene augmentation through increasing the number of copies of the gene or through a higher level of expression of the introduced gene.
All these methods used to correct defective gene is called gene therapy. Out of all these methods, targeted gene modification for gene correction or gene augmentation by introducing normal foreign gene sequences are being widely used in gene therapy.
Several reports are already published where targeted gene modification has been demonstrated in mammalian systems. The genes are introduced by gene transfer methods such as electroporation, transfection etc., followed by site specific mutagenesis e.g. HGPRT locus and int-2 loci in mouse embryonal stem cell. In past few years, approved gene therapy experiments have been conducted of which the transfer of Neo R/TIL gene marked immune cells into patients with advanced cancer was successful. ADA gene therapy used for treating the deficiency of adenosine deaminase deficiency (ADA) involved the transfection with lymphocytes bearing the ADA gene carried by retroviral vector. In Cancer gene therapy, Tumour necrosis factor gene (TNF) or IL-2 gene is inserted in TIL tumour cells isolated from the patients and grown in culture. After this these gene corrected cells are injected into the body of the patient.
In gene augmentation method, a number of copies of the desired gene are introduced and are made to express at high level using expression and transfer vectors. After the gene correction at cellular level, the implantation of the modified cells into a suitable region in an organ of the patient or in the embryo is carried out. Therefore the gene therapy can be used at two different levels: a) embryo therapy and b) patient therapy.
Embryo therapy
In this, the genetic constitution of embryo at post-zygotic level is altered which leads to the alteration in inheritance as well. Embryo therapy involves the following steps which have been tried in case of mouse or rabbit only:
a) in vitro fertilization of the egg.
b) Insertion of normal gene into embryo at post-zygotic level either using viruses or directly by microinjection.
c) Integration of inserted gene in host DNA, where it may or may not function.
The therapeutic newly inserted gene may or may not function under normal control in the animal, in time, space and quantity.
Patient therapy
In patient therapy the cells with healthy genes are introduced in the affected tissue but the inheritance trait of the patient is not affected or altered. This therapy involves the following steps:
a) Identification of defective gene
b) Isolation or synthesis of normal healthy gene.
c) Isolation of the cells of the tissue where the normal healthy gene will need to
Drawbacks of this method.
First there is the possibility that the introduced gene may not function, and the second is when corrected cells are reintroduced, these maybe outnumbered by the diseased resident cells. The other problem is that there are only few diseases affecting only a single tissue.
During the last few years, the genes are being routinely isolated. The isolated gene may either be directly injected into the cell or be carried by a virus, to which it is linked by recombinant DNA technique. After entering the cell, the gene either remains free in cytoplasm like extrachromosomal DNA or become a part of nuclear DNA . However under both circumstances only few copies of RNA are synthesized as compared to thousands of copies of RNA per cell by normal cells. Gene therapy holds a promising future and hope for patients suffering from diseases which can be treated using this technique.